| Names | |
|---|---|
| IUPAC name | |
Other names
| |
| Identifiers | |
| |
| ChEMBL | |
| ChemSpider |
|
| ECHA InfoCard | 100.002.201 |
| EC Number | |
PubChemCID | |
| RTECS number |
|
| UNII | |
CompTox Dashboard(EPA) | |
| |
| |
| Properties | |
| C4H8N2O2 | |
| Molar mass | 116.120 g·mol−1 |
| Appearance | White/Off White Powder |
| Density | 1.37 g/cm3 |
| Melting point | 240 to 241 °C (464 to 466 °F; 513 to 514 K) |
| Boiling point | decomposes |
| low | |
| Structure | |
| 0 | |
| Hazards | |
| Main hazards | Toxic, Skin/Eye Irritant |
| Safety data sheet | External MSDS |
| GHS pictograms | |
| GHS Signal word | Danger |
| H228, H301 | |
| P210, P240, P241, P264, P270, P280, P301+310, P321, P330, P370+378, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Related compounds | |
| Hydroxylamine salicylaldoxime | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| verify (what is ?) | |
| Infobox references | |
Ni Dmg 2 2 Structure 1
Nickel;N-(Z)-3-nitrosobut-2-en-2-ylhydroxylamine C8H16N4NiO4 CID 5475696 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more.
May 09, 2018 But since Ni is in +2 oxidation state in the complex, the electronic configuration would be Ar 3d8 4s0. Now, dmg is a strong field ligand which causes the two unpaired electrons to get paired up. Thus there will be 4 pairs of electrons plus an empty d orbital in 3d. The dmg ligand is a bidentate ligand. According to Tschugaeff (83) Ni(DMG)^ did not react with phenyl isocyanate; and Barker (10) could not find any reaction with acetic anhydride. Barker did claim that Ni(DMG)2 was methylated by methyl iodide; hut Thilo and Friedrich (81) reported that they observed no reaction with either dimethyl sulfate or methyl iodide. Ni(DMG)^ does not. The reaction with ethylenediamine gives: Ni(en)32+(aq) The reaction with NaOH gives: Ni(OH)2(s) The reaction with EDTA gives: NiEDTA2+(aq) Write a balanced equation for each reaction. How many d electrons are there in Ni2+? For each complex ion formed, indicate the coordination number of the copper atom. Name each complex. Do your best to. The reaction with ethylenediamine gives: Ni(en)32+(aq) The reaction with NaOH gives: Ni(OH)2(s) The reaction with EDTA gives: NiEDTA2+(aq) Write a balanced equation for each reaction. How many d electrons are there in Ni2+? For each complex ion formed, indicate the coordination number of the copper atom. Name each complex. Do your best to.
Dimethylglyoxime is a chemical compound described by the formula CH3C(NOH)C(NOH)CH3. Its abbreviation is dmgH2 for neutral form, and dmgH for anionic form, where H stands for hydrogen. This colourless solid is the dioxime derivative of the diketone butane-2,3-dione (also known as diacetyl). DmgH2 is used in the analysis of palladium or nickel. Its coordination complexes are of theoretical interest as models for enzymes and as catalysts. Many related ligands can be prepared from other diketones, e.g. benzil.
Preparation[edit]
Nickel(II) hydroxide has two well-characterized polymorphs, α and β. The α structure consists of Ni(OH) 2 layers with intercalated anions or water. The β form adopts a hexagonal close-packed structure of Ni 2+ and OH − ions. In the presence of water, the α polymorph typically recrystallizes to the β form. Jan 09, 2008 Structure of Ni(dmgH)2.As an analytical reagent, dmgH2 is often used as a solution in ethanol. It is the conjugate base, not dmgH2 itself, that forms the complexes. Furthermore, a pair of dmgH.
Dimethylglyoxime can be prepared from butanone first by reaction with ethyl nitrite to give biacetyl monoxime. The second oxime is installed using sodium hydroxylamine monosulfonate:[1]
Complexes[edit]
Dimethylglyoxime is used to detect and quantify nickel, which forms the bright red complex nickel bis(dimethylglyoximate) (Ni(dmgH)2). The reaction was discovered by L. A. Chugaev in 1905.[2]
Cobalt complexes have also received much attention. In chloro(pyridine)cobaloxime[3] the macrocycle [dmgH]22− mimics the macrocyclic ligand found in vitamin B12.
References[edit]
- ^Semon, W. L.; Damerell, V. R. (1930). 'Dimethylglyoxime'. Organic Syntheses. 10: 22. doi:10.15227/orgsyn.010.0022.CS1 maint: multiple names: authors list (link)
- ^Lev Tschugaeff (1905). 'Über ein neues, empfindliches Reagens auf Nickel'. Berichte der Deutschen Chemischen Gesellschaft. 38 (3): 2520–2522. doi:10.1002/cber.19050380317.
- ^Girolami, G.. S.; Rauchfuss, T.B.; Angelici, R. J. (1999). Synthesis and Technique in Inorganic Chemistry: A Laboratory Manual (3rd ed.). pp. 213–215.
| Names | |
|---|---|
| IUPAC name nickel;N-[(Z)-3-nitrosobut-2-en-2-yl]hydroxylamine | |
| Other names | |
| Identifiers | |
3D model (JSmol) | |
| ChemSpider | |
| EC Number | |
PubChemCID | |
| |
| |
| Properties | |
| C8H14N4NiO4 | |
| Molar mass | 288.917 g·mol−1 |
| Appearance | red solid |
| Density | 1.698 g/cm3 |
| Hazards | |
| GHS pictograms | |
| GHS Signal word | Warning |
| H315, H317, H319, H335, H351 | |
| P201, P202, P261, P264, P271, P272, P280, P281, P302+352, P304+340, P305+351+338, P308+313, P312, P321, P332+313, P333+313, P337+313, P362, P363, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
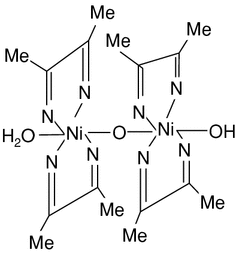
Nickel bis(dimethylglyoximate) is the coordination complex with the formula Ni[ONC(CH3)C(CH3)NOH]2. The compound is a bright red solid. It achieved prominence for its use in the qualitative analysis of nickel.[1]
Structure[edit]
Nickel(II) is square planar.[2] It is surrounded by two equivalents of the conjugate base (dmgH−) of dimethylglyoxime (dmgH2). The pair of organic ligands are joined through hydrogen bonds to give a macrocyclic ligand. The complex is distinctively colored and insoluble leading to its use as a chelating agent in the gravimetric analysis of nickel.
Ni Dmg 2 2 Structures
The use of dimethylglyoxime as a reagent to detect nickel was reported by L. A. Chugaev in 1905.[3]
References[edit]
Ni Dmg 2 2 Structure 2
- ^Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN978-0-08-037941-8.
- ^Donald E. Williams, Gabriele Wohlauer, R. E. Rundle (1959). 'Crystal Structures of Nickel and Palladium Dimethylglyoximes'. J. Am. Chem. Soc. 81: 755–756. doi:10.1021/ja01512a066.CS1 maint: uses authors parameter (link)
- ^Tschugaeff, Lev (1905). 'Über ein neues, empfindliches Reagens auf Nickel' [About a new, sensitive reagent on nickel] (PDF). Berichte der deutschen chemischen Gesellschaft (in German). 38 (3): 2520–2522. doi:10.1002/cber.19050380317.